CHEM 1001/CHM1020/CHM1025C/CHM1032C††† Name: __Answers___
Module Three Sample Pretest
Module
Three: Part D††††††† Electron Dot
Formulas†††††††† 10 points
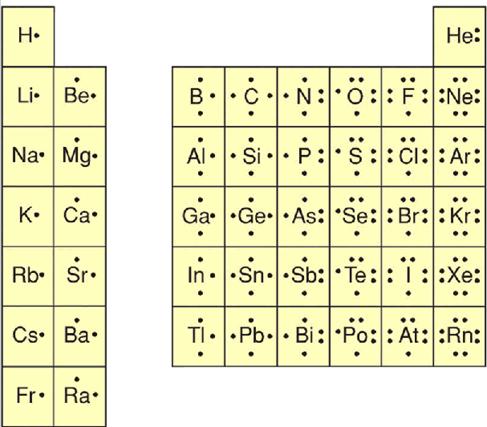
Using
the periodic chart, draw the electron dot formulas of the following elements (the
numbers shown are the elementís atomic number and mass number):
|
1.† 6C12 ††††††† 2.† 14Si28 ††††††† 3.†† 9F19 ††††††† 4.† 11Na23 ††††††† 5.† 15P31 ††††††† 6.†† 1H1 7.†† 7N14 8.†† 8O16 9.†† 10Ne20 10.† 16S32 |
Complete Table of
Representative Elementsí Dot Structures |
†††††††††††††††††† †
Module
Three: Part F†† ††††Periodic Ionic Properties††††††† 10 points
Using
a periodic chart, write the ionic character (monoatomic
ionic charge) of the following elements: (The number before the element is its atomic
number)
1.† 19 K†††† _1+_____†††††††††††††††††††††††† 6.
†††9F††††† __1-__
2.† 20Ca††† _2+____†††††††††††††††††††††††††† 7.† ††1H††††† __1+__††† __1-___††
3.† 7N††††††† _3-____†††††††††††††††††††††††††† 8.††† 16S†††† __2-__
†
4.† 17Cl††††† _1-____†††††††††††††††††††††††††† 9.††† 10Ne†† __0___
5.† 53I ††††††† _1-____†††††††††††††††† ††††††† 10.† †15P†††† __3-___††††††††