CHEM 1001/CHM 1020/ Chm1025C/CHM1032C Name:_Answers_
Module 5 SamplePretest
Part
I Mole-Mole Stoichiometry 5
points
Tungsten occurs in
the important mineral sheelite (Calcium tungstate), which is converted to tungstic
acid. Tungsten is then extracted from tundstic acid by the following (unbalanced) reaction:
3 H2 +
H2WO4 à W +
4
H2O
How moles of
hydrogen are needed to prepare 6 moles of elemental tungsten?
Question: ____?_____moles
H2 = 6 moles W

Phosphoric acid can
be made by the following (unbalanced) reaction:
6
H2O + P4O10 à 4 H3PO4
How many moles of
Phosphoric acid can be prepared from the combination of 5 moles of Tetraphosphorus decoxide with
excess water?
Question: ____?_____moles
H3PO4 = 5 moles P4O10

Suggested Additional
Homework Problems: p276 #7-12
--------------------------------------------------------------------------------------------------------------------
CHEM 1001/CHM 1020/ Chm1025C/CHM1032C Name:__Answers______
Module 5 SamplePretest
Module
Five-Part J Mass-Mass Stoichiometry 5
points
1. Toluene and
nitric acid are used in the production of trinitrotoluene (TNT), an explosive:
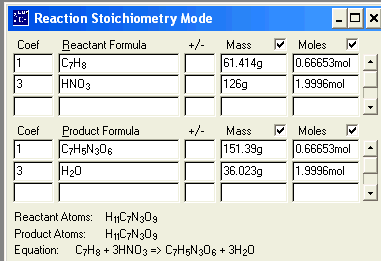
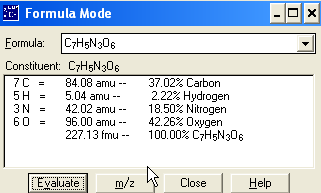
C7H8 + 3 HNO3 à C7H5N3O6 +
3
H2O (Unbalanced)
Calculate the mass
of TNT that can be made from excess C7H8 (toluene) with 126 grams of Nitric acid.
ANSWER:
Question: ____?____g C7H5N3O6
= 126 g HNO3
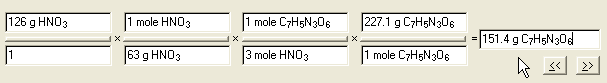
2. What mass of
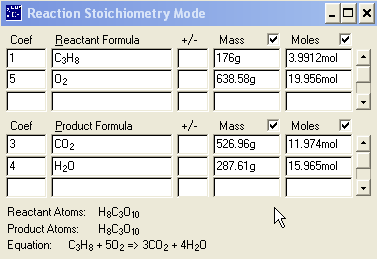
carbon dioxide is produced from the combustion of 176 grams of propane gas , C3H8
, in excess oxygen gas, O2. Water is the only other product. Write and
balance the equation for combustion.
ANSWER:
C3H8 + 5 O2 à 3 CO2 +
4 H2O
Question:
_____?_____g
CO2 = 176g C3H8

Using ChemiCalc
ANSWER:
Suggest Additional
Homework Problems: p276 #19-28