CHEM 1001/CHM 1020/ Chm1025C/CHM1032C Name:______________
Module Five SamplePretest
Part B: Mole calculation (Sections
9.2 & 9.4) 5 points
Calculate the mass in grams of 1.16 x 1022
molecules of nitrogen gas, N2
Answer: 5.39 g N2
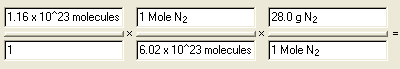
Sample Problems in text: p 247 17-22
Optional Homework: 2.5 points paper and pencil
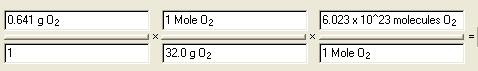
Calculate the number
of molecules in 0.641 g of oxygen gas, O2
Answer: 1.21 x 10 22 molecules O2
Sample Problems in text: p 247 5-12
Optional Homework: 2.5 points paper and pencil
Part
C: Percent Composition (section 9.7) 5 points
Calculate the
percent composition of each element in the following compound:
 Answers:
Answers:
Acetic Acid HC2H3O2

Sample Problems in the text: p
248 #39-46
Optional Paper & Pencil:
Homework 2.5 points
Part
D: Empirical Formula Calculation (Section 9.8) 5 points
Given the following
percent compositions, determine the empirical formula of the following
compound: 54.5% carbon, 9.15% hydrogen, and 36.3% oxygen
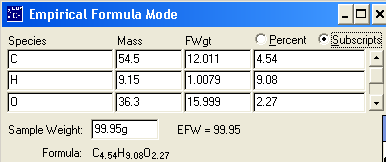
Answer: C2H4O1
Sample Problems in the text: p
248-249 #47-56
Optional Paper & Pencil:
Homework 2.5 points