Syllabus: CHEM 1001 Introductory
Chemistry for Non-Majors
Spring 2006 Section: [4] Time/Day:

Instructor:
Mr. John Taylor
Instructor’s Office: Science 202
Office Phone: (318) 427- 44357 Cell Phone: (813) 361-4379 (weekends)

email: jtaylor@lsua.edu (alternate email if
lsua is down: jtaylor@hccfl.edu )
MAPS Division Office: 473-6591
DESCRIPTION: Introductory Chemistry for Non-Science Majors
.Lec.
3 Lab. 0 Cr. 3 Prerequisite:
OBJECTIVES:
- Understanding the fundamentals of chemistry as
presented in the topical outline.
- Develop critical thinking and problem solving
skills.
- Be able to read and use data presented in graphs
and charts.
DETAILED TOPICAL OUTLINE:
1. The
scientific method and its applications
a. The steps of the scientific method
b. Theories and Laws
2. The
metric system and unit conversion
a. Making and interpreting measurements
b. Dimensional Analysis
c. Significant figures
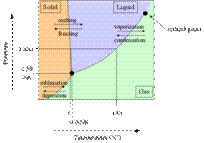
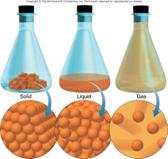
3. States
of matter
a. solid, liquids and gases
b. Laws of matter
4. Atomic
Structure
a. The atom
b. The periodic table
c. Chemical reactivity
5. Compounds
and molecules
a. Elements
b. Forming and naming compounds
6. Chemical
reactions
a. The mole
b. Balancing and stoichiometry
c. Types of chemical reactions
7. Gas
behavior
a. The combined gas law
b. The ideal gas law
8. Chemical
bonding
a. Ionic bonding
b. Covalent bonding
c. Lewis dot structure
9. Chemistry
of liquids and solids
10. Solution
chemistry
11. Acids
and bases
a. Definition of an acid and base
b. pH scale
12. Equilibrium
13. Organic/Bio
chemistry
14. Nuclear
chemistry
a. Types of particles
b. Nuclear
decay
TEXTS:
Introductory Chemistry, C.H. Corwin, 4th Edition;
Introductory Chemistry Study Guide (Optional)
 You may
use 2nd or 3rd Used Editions to save Money IF???
You may
use 2nd or 3rd Used Editions to save Money IF???
Web Site:
http://www.prenhall.com/corwin/
(Visit amazon.com or other book sellers for used copies—text +$130 in bookstore
|
Table of Contents |
|
1.1 Evolution of Chemistry. 1.2. Modern Chemistry. 1.3
Learning Chemistry. Summary. Key Concepts. Key Terms. Exercises. 2.1. Uncertainty in Measurements. 2.2. Significant Digits.
2.3. Rounding Off Nonsignificant Digits. 2.4. Adding and Subtracting
Measurements. 2.5. Multiplying and Dividing Measurements. 2.6. Exponential
Numbers. 2.7. Scientific Notation. 2.8. Unit Equations and Unit Factors. 2.9.
Unit Analysis Problem Solving. 2.10. The Percent Concept. Summary. Problem
Solving Organizer. Key Concepts. Key Terms. Exercises. 3.1. Basic Units and Symbols. 3.2. Metric Conversion
Factors. 3.3. Metric-Metric Conversions. 3.4. Metric-English Conversions.
3.5. Volume by Calculation. 3.6. Volume by Displacement. 3.7. The Density
Concept. 3.8. Temperature. 3.9. Heat and Specific Heat. Summary. Problem
Solving Organizer. Key Concepts. Key Terms. Exercises. Cumulative Review:
Chapters 1-3. 4.1. Physical States of Matter. 4.2. Elements, Compounds,
and Mixtures. 4.3. Names and Symbols of the Elements. 4.4. Metals, Nonmetals,
and Semimetals. 4.5. Compounds and Chemical Formulas. 4.6. Physical and
Chemical Properties. 4.7. Physical and Chemical Changes. 4.8. Conservation of
5. Models of the Atom. (Exam #2) 5.1.
6.1. Classification of Elements. 6.2. The Periodic Law
Concept. 6.3. Groups and Periods of Elements. 6.4. Periodic Trends. 6.5.
Properties of Elements. 6.6. Blocks of Elements. 6.7. Valence Electrons. 6.8.
Electron Dot Formulas. 6.9. Ionization Energy. 6.10. Ionic Charges. Summary.
Key Concepts. Key Terms. Exercises. Cumulative Review: Chapters 4-6. 7.1. Classification of Compounds. 7.2. Monoatomic Ions.
7.3. Polyatomic Ions. 7.4. Writing Chemical Formulas. 7.5. Binary Ionic Compounds.
7.6. Ternary Ionic Compounds. 7.7. Binary Molecular Compounds. 7.8. Binary
Acids. 7.9. Ternary Oxyacids. Summary. Nomenclature Organizer. Key Concepts.
Key Terms. Exercises. 8.1. Evidence for Chemical Reactions. 8.2. Writing
Chemical Equations. 8.3. Balancing Chemical Equations. 8.4. Classifying
Chemical Reactions. 8.5. Combination Reactions. 8.6. Decomposition Reactions.
8.7. The Activity Series Concept. 8.8 Single-Replacement Reactions. 8.9.
Solubility Rules. 8.10 Double-Replacement Reactions. 8.11. Neutralization
Reactions. Summary. Key Concepts. Key Terms. Exercises. Cumulative Review:
Chapters 7-8. 9.1. Avogadro's Number. 9.2.
10.1. Interpreting a Chemical Equation. 10.2. Mole-Mole
Relationships. 10.3. Types of Stoichiometry Problems. 10.4. Mass-Mass
Problems. 10.5. Mass-Volume Problems. 10.6. Volume-Volume Problems.
11.1. Properties of Gases. 11.2. Atmospheric Pressure.
11.3. Variables Affecting Gas Pressure. 11.4. Boyle's Law. 11.5. Charles'
Law. 11.6. Gay-Lussac's Law. 11.7. Combined Gas Law. 11.8. The Vapor Pressure
Concept. 11.9.
12.1. The Chemical Bond Concept. 12.2. Ionic Bonds. 12.3. Covalent Bonds. 12.4. Electron Dot Formulas of Molecules. 12.5. Electron Dot Formulas of Polyatomic Ions. 12.6. Polar Covalent Bonds. 12.7. Nonpolar Covalent Bonds. 12.8. Coordinate Covalent Bonds. 12.9. Shapes of Molecules. Summary. Key Concepts. Key Terms. Exercises.
13.1. Properties of Liquids. 13.2. The Intermolecular Bond
Concept. 13.3. Vapor Pressure, Boiling Point, Viscosity, Surface Tension.
13.4. Properties of Solids.
14.1. Gases in Solution. 14.2. Liquids in Solution. 14.3.
Solids in Solution. 14.4. The Dissolving Process. 14.5. Rate of Dissolving.
14.6. Solubility and Temperature. 14.7. Unsaturated, Saturated, and
Supersaturated Solutions. 14.8. Mass Percent Concentration. 14.9. Molar
Concentration. 14.10 Dilution of a Solution. 14.11.
15.1. Properties of Acids and Bases. 15.2. Arrhenius Acids
and Bases. 15.3. Bronsted-Lowry Acids and Bases.
16.1. Collision Theory. 16.2. Energy Profiles of Chemical
Reactions. 16.3. The Chemical Equilibrium Concept. 16.4. General Equilibrium
Constant, Keq. 16.5. Gaseous State Equilibria Shifts. 16.6. Ionization
Equilibrium Constant, Ki. 16.7. Weak Acid-Base Equilibria Shifts. 16.8.
18.1. Natural Radioactivity. 18.2. Nuclear Equations. 18.3. Radioactive Decay Series. 18.4. Radioactive Half-Life. 18.5. Radionuclide Applications. 18.6. Artificial Radioactivity. 18.7. Nuclear Fission. 18.8. Nuclear Fusion. Summary. Key Concepts. Key Terms. Exercises.
19.1. Hydrocarbons. 19.2. Alkanes. 19.3. Alkenes and Alkynes. 19.4. Arenes. 19.5. Hydrocarbon Derivatives. 19.6. Organic Halides. 19.7. Alcohols, Phenols, and Ethers. 19.8. Amines. 19.9. Aldehydes and Ketones. 19.10 Carboxylic Acids, Esters, and Amides. Summary. Key Concepts. Key Terms. Exercises.
20.1. Biological Compounds. 20.2. Proteins. 20.3. Enzymes. 20.4. Carbohydrates. 20.5. Lipids. 20.6. Nucleic Acids. Summary. Key Concepts. Key Terms. Exercises.
A. The Scientific Calculator. B. Weights and Measures. C. Physical Constants. D. Activity Series of Metals. E. Solubility Rules for Ionic Compounds. F. Vapor Pressure of Water. G. Properties of Water. H. Answers to Key Concept Exercises. I. Answers to Key Term Exercises. J. Answers to Odd-Numbered Exercises. Glossary. Photo Credits. Index. |
EQUIPMENT NEEDED: A scientific
calculator, periodic chart
ATTENDANCE:

Students
are expected to attend class and will be responsible for all material
presented. The student must sign the attendance roster to earn credit for
attendance. Each class attended will be
worth one point, except the first 2 points for 30 total points (3 %) of the
final grade. For each class missed 20 points of additional homework will makeup
the single point. The student will fill out a data card similar to your
instructor one the last page of this syllabus worth one point of the two points
for the first day’s attendance. Also counted in the attendance grade is the
completion of several online activities before the second week of class worth
one point each: Discovery Wheel; Interactive Time Chart; Myers Briggs Type
personality profile; Your life line, and possibly (not available) a learning
styles assessment. These may be found at:
http://www.hccfl.edu/faculty/john_taylor/cgs1555/spring04/syllabus/activity.htm
Homework: The sample pretest quizzes posted on the grading outline are not
homework to be turned. They are for the student’s self practice and for the
student to understand what the instructor expects from each section of the
textbook and his lectures. The Pretest is an actual page of a previous exam.
The grading outline may be found at: http://www.lsua.us/chem1001/01grdS06.htm
The instructor has links to online homework which the student will
complete and submit electronically on the homework outline form:
http://www.lsua.us/chem1001/01hwkS06.htm
The instructor describes paper and pencil homework for some
sections not available on the Internet on the sample pretests and/or on the
homework grading outline. The student is to keep this homework in a notebook and/or
a folder. This notebook/folder may be requested at anytime to be turned in on
exam days. Homework is to be completed prior to an exam day. The student will
grade her/his own homework and keep the homework scores on the homework grading
outline in the homework folder. Sometimes the instructor will request only that
exam’s grading form in order to post the homework scores on D2L for the Exam’s
modules.
The homework outline has
more than 70 possible points, but only 70 points maximum may be earned for no
more than 7% of the final grade. If e-Instruction is not utilized during the
classes, the homework total will expand to 120 points or 12% of the final
grade. 5% E-Instruction +7% Homework = 12% total
The first 5 points of electronic
homework is to practice spelling the elements at:
http://www.hccfl.edu/faculty/john_taylor/elementquiz/elementnew.html
Daily
Pretest Quizzes (optional):
Pretest quizzes may be administered before, during, and/or after every class which is not a scheduled exam day. These pretest quizzes may not be made up outside of class time, unless directed by the instructor to complete the pretest in the test center during an assigned period of time. Scored pretest quizzes are NOT recorded in the instructor’s grade book or on D2L, but must be attached to the Modular Exam the day of the exam to receive the pretest grade. The student will skip the section of the modular exam that is pre-tested successfully and mark the score on the first page’s test outline. The Pretest scores may be recorded on the attendance sheet, but only for your instructor’s sense of current levels of class achievement. The instructor only records Module Exam totals and the Final Exam in his grade book and on D2L. Multiple choice and vocabulary sections of modules are only tested on exam day and are never pre-tested or post-tested.
Do Not Staple the two Modular Exams together as they are
graded separately, listed on D2L separately, and returned separately after the
exam day. Please staple carefully as directed. Mixing the modular papers on
Exam day may result in a lower grade. The pretests may NOT be used during the exam!
Samples of each section (pretest) of each exam
may be found on the grading outline on the web site.
Pre-testing is a privilege not a right!
This class has no scheduled class
in the room prior to class at
E-Instruction (option):



During a scheduled class, after going through the lecture on
the assigned chapters via many modalities of teaching including Internet web
sites, the instructor will utilize either the last 10 minutes or the first 10
minutes of class to go through the power point for the assigned chapter as a
review. The power point presentation for each chapter which is posted on the
Internet menu page at:
http://www.lsua.us/chem1001/01pptmenu.html
However, multiple choice questions will be inserted into
these power points which will require all students to answer via the instructor’s
e-Instruction system (keypads). Each
correct response will be worth one point, while an incorrect response will
count zero points. e-Instruction system
will be worth no more than 50 points (out of 100 possible) for the term (5%
total) and will be included in the Homework grading total of 120 points. Each
point missed of the 50 (not the 100) will be made up by an additional 5 point
homework.
Students must read and complete their assignments before
coming to class each day. If e-Instruction
system is not used, then the homework will be expanded back to 120 total
points. Students are expected to get 50% correct on each day’s e- Instruction
questions. During the term, the instructor may pretest a section of the
multiple choice for the course using the e-Instruction system where the
responses will count 1 point each of the 10 to 15 points assigned to multiple
choice for that Module.
Major Exams:
Four exams will be administered
in class on the approximate exam days listed below. Each exam is a minimum of
two modules. Exam#4 is composed of portions of many modules. These exams will
constitute 60% of the student’s final grade or 600 points total. The grading
outline for these exams may be found at: http://www.lsua.us/chem1001/01grdS06.htm
Exams (Approximate
Date):
Placement: ACS California Exam (Week 1: T: Jan 17)
Exam
1 (Week 4: Th, Feb 9): Chapters 1-4 (Modules 1, 2)
Exam
2 (Week 8: Th, Mar 9): Chapters 5-7, 12.1-12.5 (Modules 3,4.I)
Exam
3 (Week 12: T, Apr 4): Chapters 8-11 (Modules 5, 6)
Exam
4 (Week 16: T, May 2): Chapters 12.6, 19, 13-14, 15-16, 18 (Modules 4.II, 4.III, 7,
8, 10,11, 15)
Posttest: ACS California Exam (Week
16: Th: May 4)
Final
Exam (Week 17: Tuesday, May 9
ACS California
Placement Exam:
During the first week of class the student will complete an ACS
Chemistry placement test (44 questions-50 minutes) and then on the last
scheduled day of class the same test will again be administered. A student
scoring over 22 on the pretest the first week will earn one bonus for each
correct answer above 22 (4 incorrect minus
one correct-no penalty for leaving blank for I do not know). On the last
day of the class, the Post testing will count 50 total points (5%) of the final
exam grade based on the percentile rank divided by 2. Percentile ranks are
included in the norms of the exam and the instructor will email the class with
the percentile ranks prior to the last day of class. Students missing this pre
and post test must schedule this exam in the test center within five days of
the class missed. Missing the pretest will count as a minus 50 points on the final grade.
Missing the post test will also count as a minus 50 point penalty in addition to 50 points
it counts on the final.
Final Exam:
During the final exam week, the student will complete three portions of
the final exam worth 250 total points or 25% of the final grade. Students with
an A average grade going into the final MUST take the final. No student is excused from the final.
The first portion of the
final is the ACS Chemistry placement test completed the last day of scheduled
class as described above.
The second portion is an
electronic cooperative pre-final to be completed with a student partner on a
computer connected to the Internet anytime prior to the In-class portion of the
final. The Pre-final is Closed book but open partner with the same score for
both. (It may be taken alone with permission of the instructor). It is designed
as a study tool for the comprehensive in-class final. This must be completed
prior to the in-class test. If not completed prior to the exam, then the
in-class portion will count an additional 50 points. This online test will be 150 questions for a total of 50 total points
of the final exam grade (Currently 25 Questions from exam#4
are not posted).
Pre-final/Cooperative Final
Menu: http://www.lsua.us/chem1001/01finalmenu.html
(Fall 2005 Pretest)
The third portion of the
final exam will be completed in class as scheduled by the final exam schedule.
It will be an 80 to 120 question multiple choice comprehensive final exam
during the 120 minute final exam period as designated by the published LSUA
final exam schedule. This exam will count 150 total points (or 15%) of the
final grade. If a student performs poorly on this portion of the final exam which
lowers the final grade by at least one grade less than the modular exam
average, the student may be post tested at the option of the instructor. This post
test will be completed in the test center the final day of finals, May 12 and
will be a completely new exam.
Old Finals prior to Fall may be found at: http://www.lsua.us/chem1001/01testmenu.html
Final Exam Challenge:
If the student score 180 total points out of 200 points on portions one
and three of the final (NOT the online portion), the student will receive an A
final grade in the course. If the student scores between 160 and 179 total
points the student’s final grade may be raised to a B if confirmed by the
instructor via group email prior to the last week of scheduled classes.
Post-Testing:
The instructor may post test
sections of the modular exams that a majority of the students miss. Multiple
choice and vocabulary sections will not
be pre-tested or post-tested. This post testing will be done in the test center
in a time frame established by the instructor via group email. The post test is
a free attempt. Scoring lower on the post test than on the modular exam section
will not penalize the student. The post test will be ignored and the exam
section score will count. Improving on the post test will replace that section’s
score on the modular exam. The student will resubmit his/her exam with the
graded post test stapled on top for an adjustment in the modular exam score.
MAKE-UP POLICY:
Make-up exams are usually not given. In the event of an unavoidable
absence (jury duty, hospitalization, incarceration, and death in the immediate
family), you will be allowed make-up. You must contact the instructor, no later
than, the day of the exam in order to discuss what arrangements might be made.
This may be done with a quick email. A
message must be left on the instructor's e-mail (jtaylor@lsua.edu ) if the instructor cannot
be reached. If a makeup is allowed, it must be completed prior to return of the
exam papers completed by the student attending the scheduled exam. Missed exams
will otherwise count as 0 points.
The instructor will discuss with the class those that are sick with colds,
flu, and other common illnesses which will hinder their performance on an exam.
On an individual basis he may allow make-up in the test center on exam days.
Also sick children, car and transportation problems will be dealt with on an
individual basis as well as those that just panic on test days or have
back-to-back exams on the same day. But
the rule is generally no makeup on exam day except for the instructor’s
discretion . Student abuse of absences on exam day may result in strict
enforcement of the no-makeup policy with only the unavoidable exceptions above
allowed.
Students who takes the test on the assigned test day are guaranteed to receive their graded exam on or before the next exam day after completion of the new exam, otherwise the student will be assigned a 100% grade for the un-graded paper. Student not taking the exam on exam day, may not receive their grade until days or weeks after the class papers are returned.
LSUA has a testing center.
It is located in the Student Center-Room 204. The web site for the center is: http://testing.lsua.edu/ . To use the
testing center for makeup, the student must call for an appointment at (318)
427-4492 and speak with Robin Arnold. You may also email her at ranold@lsua.edu to also setup an appointment.
Your instructor must first place the exam in the TC before you arrange an
appointment. Watch your email for makeup directions as they will change from
Module to Module.
GRADING:
Exams mainly determine a
student's letter grade. There will be 1000 points possible in the course. The four
hourly exams are worth 100-200 points (150 point average) each for a total of 600
points. The Placement test is worth 50
points, the pre-final 50 points and the comprehensive final exam is worth 150
points. The approximate grade distributions are:
900 - 1000 points = A Final Exams 25%
800 - 899 points = B Four Exams 60%
700 - 799 points = C
Homework 12% (online, notebook, e_instruction)
600 - 699 points = D
Attendance 3%
The instructor reserves the right to make necessary modifications or
adjustments to the syllabus and grading during the semester as necessary,
except that the four % distributions will not be changed: 60% Tests, 25% Final
Exam Activities, 12% Homework and 3% attendance, but the total points may vary
or other factors inserted to maintain the % distributions.
The instructor will not drop
the lowest test grade. Don’t ask! Instead a student may prove comprehension of
the material at a later time through post testing as arranged with the
instructor. A student making an A up to the final MUST take the final to earn a
final grade of A, etc.
Exams will be based on
material covered in the lecture as well as reading assignments outlined on the
course calendar and grading outline. The course calendar may be found at: http://www.lsua.info/chem1001/01calendarS06.html
Stated on the course
calendar, grading outline samples and/or worksheets/handouts.


WEB-SITE:
This course uses the lsua.us or lsua.info
web site giving you access to course information. This course also uses Desire2Learn (D2L) for group Email, to
list the Modular and Final Exams scores, and check-your-final grade through the
Internet (Note: The course materials are not currently on D2L) Access the D2L web site at: http://lsua.edu. Your username is your first,
middle and last initial (all in caps) followed by the last 4 digits of your
student ID number. Your password is your student identification number. The lsua.info or lsua.us does not require a password to sign in.
Important Course Links:
The Home
Page for this course may be found at:
http://www.lsua.us/chem1001.html
The Grading/Topic
Outline may be found at:
http://www.lsua.us/chem1001/01grdS06.htm
Links to Sample pages
of each exam are on this Grading Outline.
The power
point menu may be found at:
http://www.lsua.us/chem1001/01pptmenu.html
Pre-final/Cooperative
Final Menu:
http://www.lsua.us/chem1001/01finalmenu.html
Link to
Corwin Textbook Web Site for chapter Multiple Choice Homework:
http://www.prenhall.com/corwin/
Old Final
Menu Page (practice for Modular Exams) Homework points:
http://www.lsua.us/chem1001/01testmenu.html
Homework
Grading Outline:
http://www.lsua.us/chem1001/01hwkS06.htm
Online
first week activities:
http://www.hccfl.edu/faculty/john_taylor/cgs1555/spring04/syllabus/activity.htm
Matter Chart Links:
http://www.hccfl.edu/faculty/john_taylor/chm1025/matterchart.html
Interactive: http://www.lsua.us/phsc1003/WebExport/matterchart/index.html
Element Homework Quiz:
http://www.hccfl.edu/faculty/john_taylor/elementquiz/elementnew.html
Create your own temperature scale:
http://www.hccfl.edu/faculty/john_taylor/mathworkshop1/frametemp.html
Online dimensional Analysis
calculator:
http://www.hccfl.edu/faculty/john_taylor/chemistry/dimanalysis/unitanalysis.html
Electron configuration Online:
http://www.hccfl.edu/faculty/john_taylor/chm1045/e_config/e-1instruct.html
OFFICIAL OFFICE HOURS:
(also Unofficial – anytime I am
in my office)
Monday:
Tuesday:
Thursday:
Friday:
Instructor’s Right to Change or Modify Grading
Procedures:
This
instructor reserves the right to make changes in this syllabus whenever he
feels it is appropriate to do so. The instructor reserves the right to modify
or change the grading progress as the course proceeds. Any additional course
assignments will substitute for deleted items.
Some may also be modified if not deleted. The instructor will not add major
examinations as a modification and maintain the four exams plus final
requirements and their percent distribution.
Students with Disabilities:
Qualified
students with documented disabilities are eligible for physical and academic accommodations
under the American Disabilities Act and Section 504 of the Rehabilitation Act
of 1973. Students requesting
accommodations should contact this professor during the first week of class with official documentation of
disability
Withdrawal Policy:
Students will be allowed to withdraw
from this class any time during the semester through
Academic Misconduct:
Academic misconduct or dishonesty such as cheating and plagiarism is not permitted. Suspected cases will be reported to the LSUA administration and may result in failure of an assignment or exclusion from the class. Also, the instructor reserves the right to reassign work to students if the instructor senses the work submitted is not the work of the student. (No questions asked-The instructor may tell the student to reattempt the work to earn the daily quiz grade or examination grade or the instructor may assign a zero if second request is made).
Classroom Etiquette:
Students are expected to conduct
themselves as adults in the classroom showing respect to their classmates. Only
persons registered for this class are permitted in the classroom. As a courtesy to the instructor and your
fellow classmates, cellular
telephones and pagers should be cut off before entering the classroom or
laboratory. Likewise, the instructor sometimes forgets to shut his down
at the beginning of class, so hopefully someone sitting close to the front may
remind the instructor with a hand gesture for him to check his phone.
Disruptive students will be asked to leave.
Studying: Chemistry
is a cumulative subject. Concepts learned in the first chapter will be applied
in the second, etc. The final exam is cumulative.
In order to do well in this course, it is essential
to study and work problems from the textbook and study guide.
The following
is a list of study suggestions
1) Read the text chapters
before the material is covered in class.
2) Take good notes and review
them daily.
3) Work all assigned homework
problems at the end of the assigned chapters.
Do not get behind!!!!!!
4) Work the practice exams that
are available on the web site without looking at the answer key. Then check
your answers.
5) Use the interactive web site
and the CD-Rom distributed after the first exam for studying.
Email Requirement:
Each student should
send the instructor an email during the first week from both your lsua email
account and an outside email account for a backup contact. Be certain you put
in subject box:
01TTh: first email
Tell me
about yourself. Why are you taking this course? Did you have high school
chemistry? When? What grades did you make? What is your highest math course
completed? Where do you live? What are your telephone numbers? What is your
external email address which can serve as a backup to LSUA assigned email.
Your first assignment:
Module 1 Part B: This is a heads up! You must know (memorize if you must) the following
elements (names and symbols) by the time Exam 1 is given. Please note that a
periodic chart will be provided for every exam which contains only the symbols,
atomic numbers, and atomic masses. Reference Table 4.3, page 79 of your text
for most of the elements listed below:
Required Elements: Atomic #s: 1-38, 46-57,
74, 76-80, 82, 83, 86-89, 92 & 94
For homework, you will
practice the spelling of the elements at:
http://www.hccfl.edu/faculty/john_taylor/elementquiz/elementnew.html
A pretest quiz will be
administered the first five minutes of the first class of the second week of
school. A hard copy of a sample quiz may be obtained at:
http://www.lsua.us/chem1001/sampletest/01M1b.htm
Also the second week of the
term you will complete Module 1 Part A, the Matter chart. The actual quiz page
may be found at:
http://www.lsua.us/chem1001/sampletest/01M1a.htm
and there is an interactive
practice at:
http://www.lsua.us/phsc1003/WebExport/matterchart/index.html



Instructor Requested Information:
During the first week of
class, the student will fill out a 4x6 file card. The instructor has provided a
sample below with his personal data and his block scheduled time. The completion of this card is worth (2 points)
toward the student's final grade
Data Card (4x6 file card): Front Side (Personal Data)
--------------------------------------------------------------------------------------
Name: John Taylor CHEM 1001
Office: Science 202
Address:
Telephone: 427-4435 (office)
Cell: 813 361-4379 (cell after
E-MAIL : jtaylor@lsua.edu or jtaylor@hccfl.edu
Employment: LSU-Alexandria since
Full time chemistry faculty
Major:
Instructional Technologies Minor:
Chemical Education
Long Term Goal:
Educational Software Developer
Prerequisite:
College Algebra completed
Chemistry
Background: High School chemistry
completed: yes
Software/Computer Literacy: WP: Word
Home Computer: yes Internet ISP: yes or have access
Why are you
taking this course? Required for nursing program
--------------------------------------------------------------------------------------
Put your class and work schedule on the back side of the data card
See next page!
Class Schedule
Number Section Room Time Days
CHEM 1001 2 Sc
203
CHEM 1001 4 Sc
203
CHEM
1001 21 Air
Park
CHEM 2414 21 Sc
203
PHSC 1001 4 Sc
118
PHSC 1001 21 Sc
203
PHSC 1003 1 Sc
208
PHSC 1003 2 Sc
208
Class/Office Matrix:
My Schedule matrix: Please
make your own. I have 10 hours of office hours, you must find 10 hours in you
weekly matrix for studying chemistry:
|
|
Monday |
Tuesday |
Wednesday |
Thursday |
Friday |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
office |
|
|
|
|
|
|
office |
|
|
CHEM1001-2 |
PHSC1001-4 |
CHEM1001-2 |
PHSC1001-4 |
CHEM1001-2 |
|
|
CHEM1001-2 |
PHSC1001-4 |
CHEM1001-2 |
PHSC1001-4 |
CHEM1001-2 |
|
|
office |
PHSC1001-4 |
office |
PHSC1001-4 |
office |
|
|
office |
|
office |
|
|
|
|
|
office |
1003-1 & 2 |
|
|
|
|
|
office |
1003-1 &2 |
office |
|
|
|
|
office |
1003-1 & 2 |
office |
|
|
|
|
office |
1003-1 & 2 |
office |
|
|
|
|
CHEM1001-4 |
1003-1 & 2 |
CHEM1001-4 |
|
|
|
|
CHEM1001-4 |
office |
CHEM1001-4 |
|
|
|
|
CHEM1001-4 |
office |
CHEM1001-4 |
|
|
|
|
|
|
office |
|
|
|
|
|
|
CHEM2414-21 |
|
|
|
|
|
|
CHEM2414-21 |
|
|
|
|
office(AP) |
Office |
CHEM2414-21 |
|
|
|
|
office(AP) |
Office |
CHEM2414-21 |
|
|
|
|
CHEM1001-21 |
PHSC1001-21 |
CHEM2414-21 |
|
|
|
|
CHEM1001-21 |
PHSC1001-21 |
CHEM2414-21 |
|
|
|
|
CHEM1001-21 |
PHSC1001-21 |
CHEM2414-21 |
|
|
|
|
CHEM1001-21 |
PHSC1001-21 |
CHEM2414-21 |
|
|
|
|
CHEM1001-21 |
PHSC1001-21 |
office |
|
|
|
|
CHEM1001-21 |
PHSC1001-21 |
|
|
|
|
|
|
|
|
|